Sterilization is a crucial process in the medical, pharmaceutical, and food industries, ensuring that products are free from harmful microorganisms. One of the most effective methods of sterilization is Ethylene Oxide (ETO) Sterilization, Ethylene oxide (ETO) sterilization is one of the most widely used methods to sterilize medical devices, pharmaceutical products, and other sensitive items that cannot withstand traditional heat-based sterilization methods. The use of ETO evolved when few alternatives existed for sterilizing heat- and moisture-sensitive medical devices; however, favorable properties (Table 6) account for its continued widespread use.872 Two ETO gas mixtures are available to replace ETO-chlorofluorocarbon (CFC) mixtures for large capacity, tank-supplied sterilizers. At INSTECH SYSTEMS, we specialize in providing high-quality ETO sterilizers that meet industry standards, ensuring safety and efficiency in various applications.
What is ETO? – Full Form of ETO
ETO stands for Ethylene Oxide, a colorless, flammable gas used in the sterilization process. Ethylene oxide is known for its ability to destroy bacteria, viruses, fungi, and other microorganisms by disrupting their cellular structure. This makes ETO an excellent choice for sterilizing heat-sensitive materials that cannot be exposed to high temperatures, such as medical devices, electronics, and some food products. ETO is a flammable and colorless gas, which is introduced in a sealed chamber and interacts with microorganisms such as virus, bacteria, fungi and spores.
The ETO Sterilizer Process
The process is particularly suited for heat-sensitive materials like plastics, rubber, and electronics. This makes ETO sterilization indispensable for items like surgical instruments, catheters, syringes, and other medical devices. ETO sterilization process involves several key steps, carefully controlled to ensure effective sterilization while maintaining the integrity of the materials being treated. Here’s an overview of how the process works:
- Preconditioning:
The items to be sterilized are first placed inside the sterilization chamber. Before introducing the ethylene oxide gas, the items are preconditioned by adjusting the temperature and humidity to levels that promote effective gas penetration. - Gas Introduction:
Once preconditioning is complete, ethylene oxide gas is introduced into the sterilization chamber. The gas is allowed to penetrate the items thoroughly, reaching all surfaces, even the most difficult-to-reach areas. The gas molecules are absorbed by the microorganisms, disrupting their cellular functions and rendering them inactive. - Exposure Time:
The materials are exposed to the ethylene oxide gas for a specific period, which can vary depending on the type of materials being sterilized, the gas concentration, and the required sterility level. The exposure time typically ranges from a few hours to a full day. - Aeration:
After the exposure phase, the sterilized items are removed from the chamber and undergo an aeration process. This step is essential to remove any residual ethylene oxide gas from the materials, ensuring they are safe for handling and use. The aeration process may take several hours, depending on the product. - Sterility Assurance:
After aeration, the sterilized items are checked for sterility. This step involves using biological indicators, such as bacterial spores, to confirm that the sterilization process has been successful and that no microorganisms remain on the items.
Benefits of ETO Sterilization
- Effective on Heat-Sensitive Materials:
ETO sterilization is ideal for materials that cannot withstand high temperatures, such as plastics, rubber, and certain medical devices, making it a preferred method in the medical industry. - Wide Range of Applications:
ETO is used across multiple industries, including healthcare (sterilizing surgical instruments, implants, and medical devices), pharmaceuticals (sterilizing drugs and packaging materials), and food processing (for sterilizing spices and herbs). - Penetrates Complex Geometries:
Unlike other sterilization methods, ETO gas can penetrate complex or intricate structures, such as porous materials and multi-component devices, ensuring complete sterilization. - Minimal Impact on Material Integrity:
Since the process is carried out at low temperatures, ETO sterilization does not damage the structure or properties of the sterilized items, maintaining their functionality and longevity. - Effective against a Wide Range of Microorganisms:
ETO sterilization is highly effective against bacteria, viruses, fungi, and spores, making it a versatile and reliable method for achieving sterility.
Critical Parameters for Effective Ethylene Oxide Sterilization
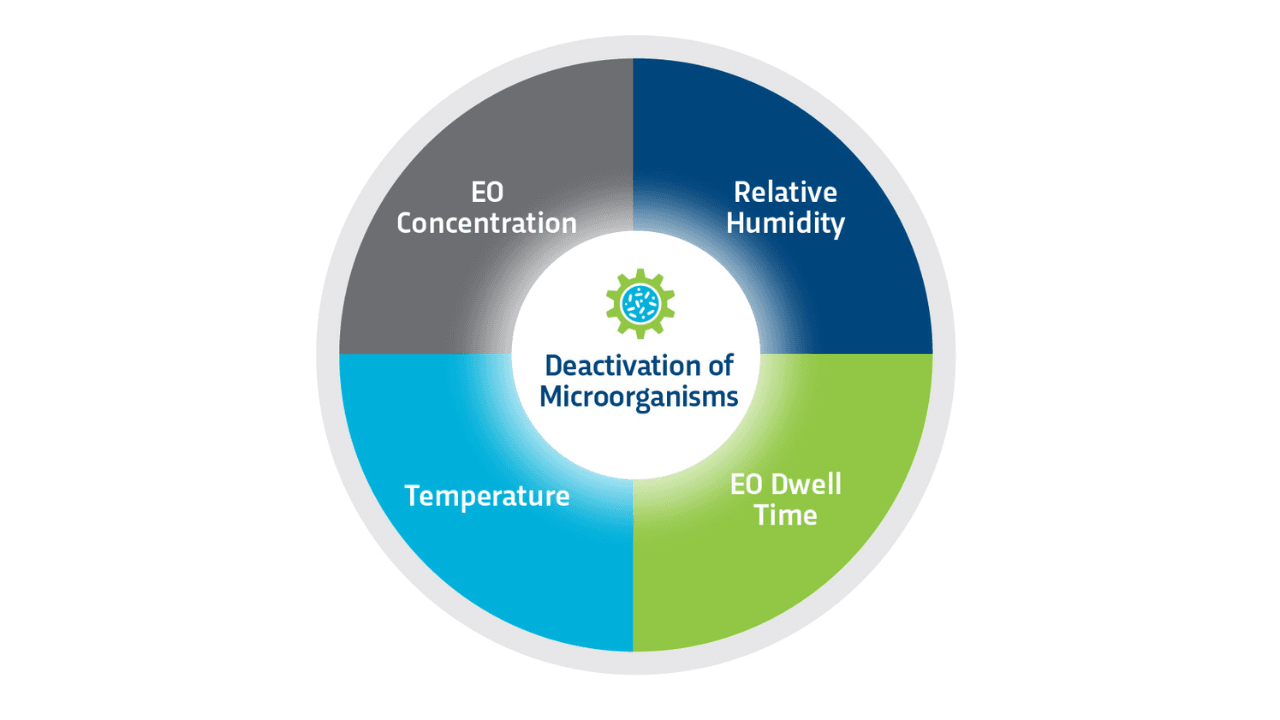
Based on a gas diffusion process, Ethylene Oxide (EO or ETO) is capable of sterilizing and rendering products free of viable microorganisms. Sterility occurs when an EO gas molecule reacts with and destroys the microbial DNA. The process requires the simultaneous control of four variables, but interdependent parameters: gas concentration, temperature, relative humidity, and time of exposure. ETO sterilization effectiveness depends on its ability to freely diffuse through a product and packaging. All products must be placed in breathable packaging that allows gas to penetrate the sterile barrier and reach all surfaces of the device or product.
ETO Sterilizer Features :-
- The machine suits Sterilize Surgical Medical Device products like I.V. Sets, Disposable Syringes, Disposable Surgical sets, catheters, Urine bags, etc.
- ETO Sterilization reduces microbiological load and increases the lifespan of material.
- Modular construction of chamber.
- The model is available from 0.5 cubic meters to 40 cubic meters.
- Easy to operate.
- Fully automatic operation based on PLC control.
- Semi-automatic (pneumatically operated Ball Valve) Control.
- Contact parts are SS 304 / 316.
- The processing time is about 8 hours.
- IQ/OQ/DQ documentation can be offered on demand. (OPTIONAL).
- It is useful for preventing microbiological contamination and treating spices, dried nuts, packaged cereals, etc.
Why Choose INSTECH SYSTEMS for ETO Sterilization Process?
At INSTECH SYSTEMS, we are dedicated to providing advanced ETO sterilization equipment that meets the highest industry standards. Our sterilizers are designed for precision, reliability, and efficiency, ensuring that your sterilization process is seamless and effective. With years of experience in the field, we offer both standard and customized solutions to suit your unique needs. We provide advanced solutions designed to meet 21 CFR Part 11 requirements. Our systems feature robust access controls, audit trails, and data encryption to maintain compliance while enhancing operational efficiency.
As a best ETO Sterilizer manufacturer, we ensure that our product performs well for ETO sterilizers Processes are equipped with advanced controls and monitoring systems to ensure that each cycle is executed precisely. Whether you are in the healthcare, pharmaceutical, or food industry, we have the expertise and technology to help you achieve optimal sterilization results. If you are looking further for a ETO sterilizer machine, pls contact us. We are always there to solve your queries and provide you more details about our product.