21 CFR Part 11
21 CFR Part 11, established by the FDA, governs the use of electronic records and electronic signatures in regulated industries. It ensures data integrity, security, and authenticity for compliance with quality and regulatory standards.
At INSTECH SYSTEMS, we provide advanced solutions designed to meet 21 CFR Part 11 requirements. Our systems feature robust access controls, audit trails, and data encryption to maintain compliance while enhancing operational efficiency.
Partner with INSTECH SYSTEMS to ensure your processes are aligned with industry standards, giving you confidence in managing electronic records and signatures securely and reliably.
21 CFR Part 11
21 CFR Part 11 is a regulation issued by the United States Food and Drug Administration (FDA) that establishes criteria for electronic records and electronic signatures. It applies to companies that create, modify, maintain, or transmit electronic records related to drug or device manufacturing, testing, and distribution.
21 CFR Part 11 refers to software systems that meet the criteria set forth in the regulation. These criteria include requirements for:
- Security: the software must have controls in place to ensure the confidentiality, integrity, and availability of electronic records.
- Audit trails: the software must be able to record and retain information about every change made to an electronic record, including who made the change and when.
- Electronic signatures: the software must have controls in place to ensure that electronic signatures are unique, cannot be transferred, and are linked to the individual signing the record.
- Validation: the software must be validated to ensure that it functions as intended and meets the requirements of the regulation.
21 CFR Part 11 is important for companies that operate in regulated industries to ensure compliance with FDA regulations and to maintain the safety and efficacy of their products. Software vendors that offer 21 CFR Part 11 compliant software typically provide documentation and support to help their customers meet the regulation’s requirements.
Instech JM.S Development and Function:
Instech JM.S includes a set of functions for responding to the requirements specified in FDA 21 CFR Part 11. The standard is intended to provide a solution for securely handling electronic records and electronic signatures in industrial applications.

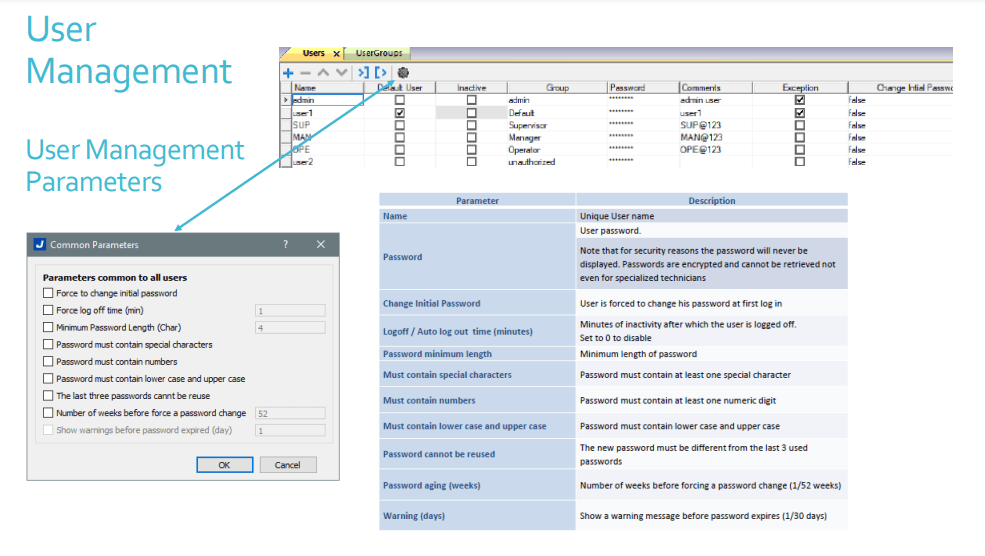
User Management Security
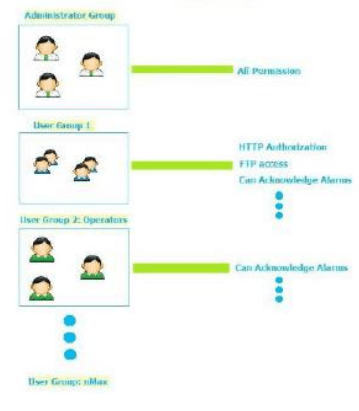
- Instech JM.S provides customizable user group dynamics
- You can restrict access to various widgets and operations by configuring users, user groups and assigning specific authorizations to each group
- Max User Groups = 50
- Max Users = 500
- Each user must be member of one and only one group. Each group has specific authorizations and permissions.
- By organizing permissions and groups you can define the security options of a project
What is the purpose of 21 CFR Part 11?
The primary purpose of 21 CFR Part 11 is to define the requirements for the use of electronic records and electronic signatures in FDA-regulated industries. It ensures that electronic records are secure, reliable, and legally binding, just like traditional paper records and handwritten signatures.
Which industries are affected by 21 CFR Part 11?
Industries that are subject to FDA regulations, such as pharmaceuticals, biotechnology, medical devices, food manufacturing, and clinical research, are typically affected by 21 CFR Part 11.
What are electronic records under 21 CFR Part 11?
Electronic records are any information created, modified, maintained, archived, retrieved, or transmitted electronically, including text files, databases, spreadsheets, and other digital formats.
What are electronic signatures under 21 CFR Part 11?
Electronic signatures are digital representations of handwritten signatures, which can be used to sign electronic records. These signatures are used to authenticate and link an individual to the electronic record.
What are the consequences of non-compliance with 21 CFR Part 11?
Non-compliance with 21 CFR Part 11 can result in regulatory actions, including warning letters, fines, product recalls, and legal consequences. It’s essential for regulated industries to ensure compliance to avoid these risks.
How can organizations achieve compliance with 21 CFR Part 11?
Achieving compliance involves implementing appropriate policies, procedures, and technologies to ensure the secure and reliable use of electronic records and signatures. This typically includes system validation, data encryption, access controls, and the establishment of audit trails.
